Траниласт, стабилизатор мембраны тучных клеток, снижает содержание мочевой кислоты в крови, подавляя основные реабсорбирующие транспортеры уратов.
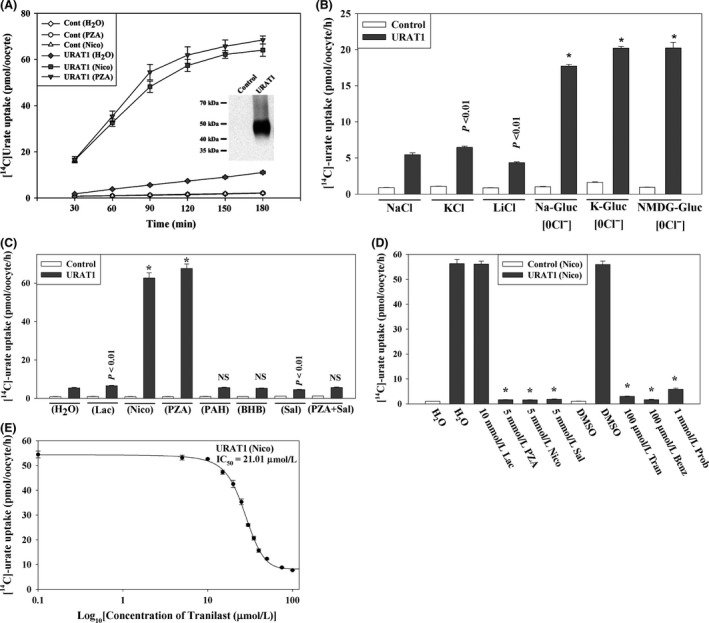
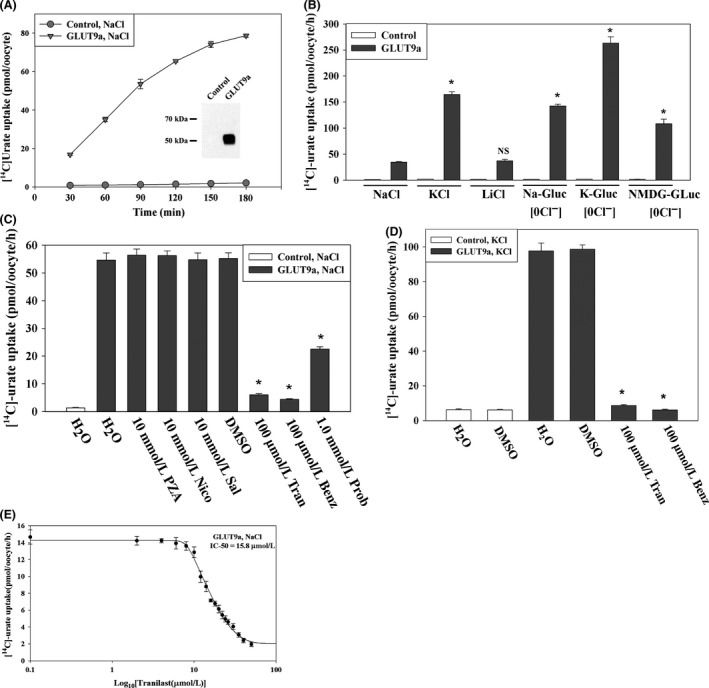
 Мочевая кислота, продукт пуринового обмена, играет как хорошо известные, так и недавно открытые роли в патогенезе различных заболеваний человека. Уровень мочевой кислоты в сыворотке человека определяется скоростью ее синтеза и разницей между реабсорбцией и секрецией в почках и кишечнике. В почках человека реабсорбция эпителием преобладает над секрецией, то есть у здоровых людей реабсорбируется не менее 90% профильтрованных уратов, а выводится менее 10%. Траниласт, противовоспалительный препарат с различными дополнительными эффектами, оказывает у человека выраженный урикозурический и, следовательно, гипоурикемический эффект. В настоящей работе мы показали, что траниласт — сильный ингибитор транспорта [14C]-уратов, осуществляемого основными реабсорбирующими транспортерами уратов (URAT1, GLUT9, OAT4 и OAT10) в яйцеклетках лягушек Xenopus, и таким образом явно показали механизм урикозурического действия препарата. Траниласт — обратимый и неконкурентный ингибитор URAT1 и GLUT9 (смешанный тип ингибирования). Кроме того, он ингибирует секреторные транспортеры уратов NPT1, OAT1 и OAT3, не влияя на секреторный эффлюксный насос ABCG2. Интересно, что бензбромарон и пробенецид подавляют транспорт как уратов, так и никотинатов, а траниласт подавляет транспорт молекулами URAT1, GLUT9, OAT4, OAT10 и NPT1 только уратов, не оказывая значимого влияния на транспорт никотинатов белками SMCT1 (IC 50 ~1,1 ммоль/л), SMCT2 (IC 50 ~1,0 ммоль/л) и URAT1 (IC 50 ~178 мкмоль/л). Итак, траниласт снижает содержание мочевой кислоты в крови, подавляя основные реабсорбирующие транспортеры уратов и не затрагивая при этом транспорт никотинатов. Эти данные могут быть полезны для лечения гиперурикемии и подагры, понимания фармакологии траниласта и структурно-функционального анализа транспорта уратов.
Мочевая кислота, продукт пуринового обмена, играет как хорошо известные, так и недавно открытые роли в патогенезе различных заболеваний человека. Уровень мочевой кислоты в сыворотке человека определяется скоростью ее синтеза и разницей между реабсорбцией и секрецией в почках и кишечнике. В почках человека реабсорбция эпителием преобладает над секрецией, то есть у здоровых людей реабсорбируется не менее 90% профильтрованных уратов, а выводится менее 10%. Траниласт, противовоспалительный препарат с различными дополнительными эффектами, оказывает у человека выраженный урикозурический и, следовательно, гипоурикемический эффект. В настоящей работе мы показали, что траниласт — сильный ингибитор транспорта [14C]-уратов, осуществляемого основными реабсорбирующими транспортерами уратов (URAT1, GLUT9, OAT4 и OAT10) в яйцеклетках лягушек Xenopus, и таким образом явно показали механизм урикозурического действия препарата. Траниласт — обратимый и неконкурентный ингибитор URAT1 и GLUT9 (смешанный тип ингибирования). Кроме того, он ингибирует секреторные транспортеры уратов NPT1, OAT1 и OAT3, не влияя на секреторный эффлюксный насос ABCG2. Интересно, что бензбромарон и пробенецид подавляют транспорт как уратов, так и никотинатов, а траниласт подавляет транспорт молекулами URAT1, GLUT9, OAT4, OAT10 и NPT1 только уратов, не оказывая значимого влияния на транспорт никотинатов белками SMCT1 (IC 50 ~1,1 ммоль/л), SMCT2 (IC 50 ~1,0 ммоль/л) и URAT1 (IC 50 ~178 мкмоль/л). Итак, траниласт снижает содержание мочевой кислоты в крови, подавляя основные реабсорбирующие транспортеры уратов и не затрагивая при этом транспорт никотинатов. Эти данные могут быть полезны для лечения гиперурикемии и подагры, понимания фармакологии траниласта и структурно-функционального анализа транспорта уратов.
Mandal AK1, Mercado A2, Foster A1, Zandi-Nejad K3, Mount DB1.
Pharmacology Research & Perspectives 2017 Feb 6;5(2):e00291.
DOI: 10.1002/prp2.291. eCollection 2017
![]()
Uricosuric targets of tranilast
Uric acid, generated from the metabolism of purines, has both proven and emerging roles in human disease. Serum uric acid in humans is determined by production and by the net balance of reabsorption and secretion in kidney and intestine. In the human kidney, epithelial reabsorption dominates over secretion, such that in normal subjects there is at least 90% net reabsorption of filtered urate resulting in a fractional excretion of <10%. Tranilast, an anti-inflammatory drug with pleiotropic effects, has a marked hypouricemic, uricosuric effect in humans. We report here that tranilast is a potent inhibitor of [14C]-urate transport mediated by the major reabsorptive urate transporters (URAT1, GLUT9, OAT4, and OAT10) in Xenopus oocytes; this provides an unequivocal molecular mechanism for the drug’s uricosuric effect. Tranilast was found to inhibit urate transport mediated by URAT1 and GLUT9 in a fully reversible and noncompetitive (mixed) manner. In addition, tranilast inhibits the secretory urate transporters NPT1, OAT1, and OAT3 without affecting the secretory efflux pump ABCG2. Notably, while benzbromarone and probenecid inhibited urate as well as nicotinate transport, tranilast inhibited the urate transport function of URAT1, GLUT9, OAT4, OAT10, and NPT1, without significantly affecting nicotinate transport mediated by SMCT1 (IC 50 ~1.1 mmol/L), SMCT2 (IC 50 ~1.0 mmol/L), and URAT1 (IC 50 ~178 μmol/L). In summary, tranilast causes uricosuria by inhibiting all the major reabsorptive urate transporters, selectively affecting urate over nicotinate transport. These data have implications for the treatment of hyperuricemia and gout, the pharmacology of tranilast, and the structure-function analysis of urate transport.
KEYWORDS:
Benzbromarone; GLUT9; URAT1; gout; hyperuricemia; tranilast; uric acid







